Faraday's Law Material: Sounds, Formulas, Example Problems
Loading...
Do you know what Faraday's Law is? One of the laws related to electromagnetism and provides predictions of interactions between electric circuits and magnetic fields so that they can produce electromotive forces.
Discovered in 1833 by British scientist Michael Faraday, until now it is still used and is very useful in certain fields. You want to know what it means and examples of questions?
List of contents
Faraday's 1st Law

There are two Faraday's Laws, the first relates to the process of electrolysis and electric charge.
It reads "The mass of the substance produced from an electrode during the electrolysis process will be directly proportional to the amount of electric charge used"
The equations used are:
W Q
W = mass of a substance
Q = electric charge of electrons
The usage is:
Q = i x t
i = electric current (in amperes)
t = time (in seconds)
With the explanation that the sum of the use of electric charges, the result will be equal to the product of the current strength with time.
Furthermore, there are more similarities from the results of the use of the law above, becoming:
w I x ta
As for the formula used in its application are:
W = e.i.t/F
Information:
W = mass of a substance produced in an electrolysis process (in grams)
e = equivalent mass
i = current strength (in amperes)
t = time (in sec)
F = Faraday's determination with a definite number of 96,500 Coulomb/mol.
The application of Faraday's law in the world of chemistry can be seen through several components related to electric current and electrolysis.
As in the electric generator, which is a special device with the function of generating electrical energy through mechanical sources and utilizing electromagnetic induction. Generators have two types, namely the current flows back and forth and direct.
The next application is a dynamo which has two types, namely alternating current and direct current.
Read: Kirchhoff's Law
Faraday's 2nd Law

In contrast to Faraday's 1st Law, there is another second with the sound "The mass of the substance produced by an electrode during the electrolysis process, will be directly proportional to the equivalent mass of the substance the.
While what is meant by the mass of a substance is, the mass of a substance that is in an application of the law. Then the equivalent mass is the mass of a substance, stoichiometrically having the same number of moles as 1 mole of an electron.
The equation is:
Mass of substance mass equivalent of substance
w ME
ME = oxidation state or charge of the ion/Ar
The formula used in the application of this second law is:
W1/W2 = e1/e2
Information:
W1 = mass of the first substance (in grams)
W2 = mass of the second substance (in grams)
e1 = equivalent mass of the first substance
e2 = equivalent mass of the second substance
In the application of this second law, the equivalent mass function is very important. Divided by the change in oxidation number that occurs in an electrolysis reaction.
The equation is:
Moak = Ar
Change in oxidation number
When referring to basic stoichiometric concepts, there is a relationship between mass, the number of mo, and the mass of the molar which will be closely related to the combined use of Faraday's 1st and 2nd laws.
The equation is:
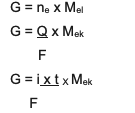
The summary is the combined equation between and 2, namely:
Advertisement
G ~ i x t x Moak
Information:
G = mass of the product (in grams)
Q = electric charge (in coulomb units)
i = strength of electric current (in amperes)
t = time (in sec)
Moak= equivalent mass of the substance (in grams/mol)
F = Faraday's constant which is 96,500 coulomb/mol)
Read: Hooke's Law
Example of Faraday's Law Problem
To better understand the application of Faraday's Law, here are some examples of its application.
1. Example 1
There has been a Cu deposition in an electric circuit with a magnitude of 5 grams. What mass of Ag has precipitated at the electrodes? For additional information Ag = 108, and Cu = 63.5.
For the formula to find the mass of Ag or WAg you can use the following formula:
WCu = MECu
WAg= MEAg
ME is known by the equation = Ar
Oxidation Number = 2
Is known:
WCu = 5 grams
First calculate the ME of the two substances by using the reduction reactions of both Cu and Ag as follows:
Cu2+ + 2e– → Cu(s), MEcu
= Ar = 63,5
Oxidation number =2
= 31,75
Ag+ + e– → Ag(s) MEAg
= Ar = 108
Oxidation number = 1
= 108
next 5gCu = 31.75
WAg 108
= 17
So the mass of Ag that settles is = 17 grams.
2. Example 2
How many Faradays will it take to reduce Ca. ions?2+ with the amount of 12 grams? Here is the discussion:
Ca2+→ the valence is 2
Ar of Ca is 40
The formula used is W = e x F
Where to find F = W/e
The answer is:
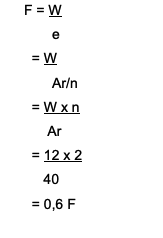
So, it takes 0.6 Faraday to reduce Ca. ions2+ which amounts to 12 grams.
Read: Coulomb's Law
3. Example 3
An electric current of 0.2 amperes is allowed to flow for a period of 50 minutes, then enters the electrolysis cell containing a CuCl solution.2. What is the amount of Cu precipitate that will form at the cathode. As additional information Ar from Cu = 63.5
Is known:
Current with symbol I = 0.2 ampere
Time with the symbol t = 50 minutes, if used as seconds is 50 x 60 seconds, which is 3,000 seconds
Asked: WCu?
Answer: First write down the Cu reduction reaction, namely:
Cu2+ (aq) + 2e+ → Cu(s)
MECu = Ar
Oxidation number = 63.5/2
= 31,75
The formula used to find Wcu is:
Wcu = (1/96,500) x i x t x MEcu
= (1/96,500) x 0.2 A x 3,000 s x 31.75
= 0.197 grams
So, the value of Cu deposition that occurs is 0.197 grams.
4. Example 4
In an electrolysis with a carbon type electrode, it was able to produce a Cu type precipitate with an amount of 12.7 grams. Uses an electric current of 4 amperes. How long will it take for the electrolysis?
Is known:
Ar of Cu = 63.5
Asked: W?
Answer:

So the time for electrolysis is 9,650 seconds using the formula from Faraday's law that applies.
For those of you who are studying Chemistry, of course you will really need a proper understanding of Faraday's law. So that when given a task related to the application of the law, you can answer it correctly and thoroughly using the formulas that are already available.
X CLOSE
Advertisements
ADVERTISEMENT
X CLOSE
