HEAT: Formulas, Types, Sample Problems (Summary)
Loading...
The heat formula is a familiar thing to find in Physics lessons. Heat itself is a form of energy that can be received or released by an object. Heat has units called joules or calories.
Heat can be interpreted as heat energy possessed by certain substances, and can be detected by measuring the temperature of the object. You can see the application of heat to warm water that is left open, gradually cooling (not warming anymore) as heat is released from the water into the air.
List of contents
Definition of Calorie

Heat is an energy that can move from an object with a higher temperature to an object with a lower temperature when the two objects touch each other or are brought together. Two objects with different temperatures will cause heat to flow and move.
For example, when you mix hot water and cold water, the water that combines will become warm water. You also have to understand that temperature and heat are two different things. Temperature is a value that can be measured with a thermometer, while heat is energy that flows.
According to the International System (SI) or MKS, the unit of heat is Joule (J), while according to CGS the unit of heat is erg. For a number of types of food, heat uses the calorie unit.
One calorie is defined as the amount of heat energy required to raise the temperature of 1 gram of water to 1 degree Celsius. So, it can be concluded that 1 calorie = 4.184 Joules or generally rounded up directly to 4.2 J.
Calorie Formula
According to the definition of heat that you have read above, the following are some of the formulas that have been summarized relating to heat material in Physics subjects.
1. Heat Transfer Formula
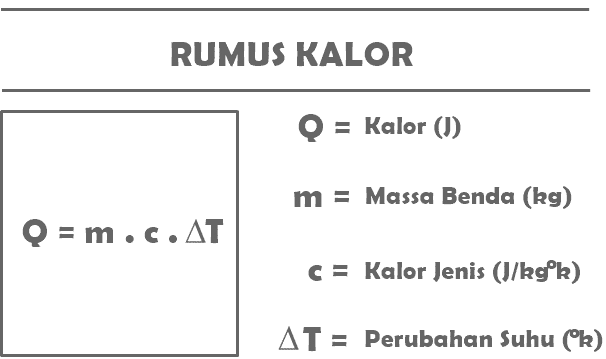
Q = m. c. T
With:
Q = the amount of heat received or released by a certain object (J)
m = mass of the object receiving or releasing heat (kg)
c = specific heat of substance (J/kg°C)
T = change in temperature (°C)
2. Specific Heat Formula
The benchmark of this formula is the heat transfer formula, by removing the element c (specific heat of substance) to be calculated mathematically as usual.
c = Q / (m. T)
With:
c = specific heat of substance (J/kg°C)
Q = the amount of heat received or released by a certain object (J)
m = mass of the object receiving or releasing heat (kg)
T = change in temperature (°C)
3. Heat Capacity Formula
C = Q / T
With:
C = heat capacity (J/°K)
Q = a lot of heat (J)
T = change in temperature (Kelvin / K)
4. Formula for Determining Heat Capacity
C = m. c
With:
C = heat capacity (J/°K)
m = mass of the object receiving or releasing heat (kg)
c = specific heat of substance (J/kg°K)
5. The formula for heat of fusion and steam
The formula for the heat of fusion is as follows.
Q = m. L
The formula for the heat of vapor is as follows.
Q = m. U
With:
Q = a lot of heat (J)
m = mass of object (kg)
L = heat of fusion (J/kg)
U = vapor heat of the substance (J/kg)
Read: Thermodynamics
Types of Calories

There are several types of heat that are distinguished based on the work process on a particular substance. Below are the types of heat that you need to understand to be able to see its application in everyday life.
1. Heat of Formation (ΔHf)
The heat of formation is the heat produced or required to form 1 mole of a compound in its elements, such as gases, which are written using the molecular formula. Some examples of heat of formation are C12, O2, Br2, H2.
2. Heat of Decomposition (ΔHd)
The heat of decomposition is the form of heat produced or required to break down 1 mole of a compound into another element.
3. Heat of Combustion (ΔHc)
The heat of combustion is the heat gained or needed to burn 1 mole of a substance, such as an element or its compound.
4. Heat of Neutralization (ΔHn)
The heat of neutralization is the type of heat obtained or required to form one mole of H2O from the reaction between an acid and a base. This heat is an exothermic reaction because there is an increase in temperature.
5. Heat of Dissolution (ΔHs)
The heat of dissolution is the type of heat produced or required to dissolve 1 mole of a substance that was originally solid into a solution.
Read: Density Formula
Specific Heat and Heat Capacity
You also need to know that heat can flow in two substances that have different particles of matter and different temperature changes. For example, when water and oil are heated to the same temperature, the temperature of the oil changes more than the temperature of the water changes.
This can happen because there are different types of heat in two objects that are brought together or put together. Specific heat is the amount of heat required to raise the temperature from 1 kg of mass to 1°C, the unit is calories/gram°Celcius or J/kg°C.
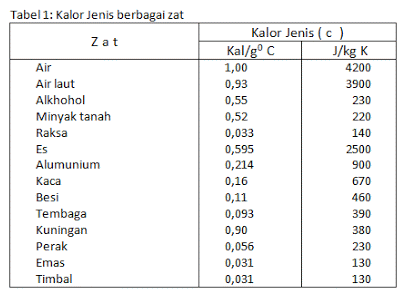
Each substance has its own specific heat and there are differences in it. Meanwhile, the notion of heat capacity is the amount of heat required or absorbed to be able to raise the temperature of an object to 1°C.
Heat Change
In its application, the heat formula works using the principle that changes two substances that are brought together or in contact with each other. Below is a heat change that occurs when there are substances that are put together or brought together.
1. Heat Can Change the Temperature of a Substance
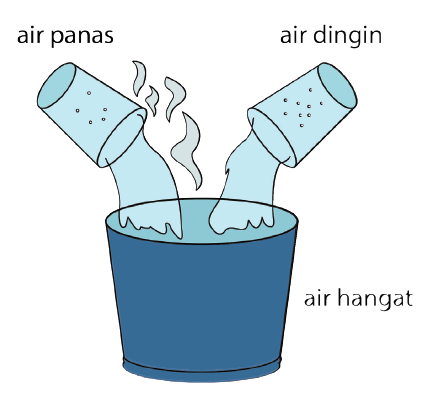
Each basic object has a temperature lower than absolute zero, so the object must have heat. This content will later determine how much heat the object's temperature has.
When the object is heated, it will get additional heat. The temperature will also increase or increase. Conversely, if the object is cooled, then heat will be released and cause a decrease in temperature.
2. Heat Can Change Substances

In some types of objects, when given heat in certain units, the object can undergo a change of state. For example, if ice is given heat (heated) then the ice will change form from solid to liquid or even gas.
If the heating process is carried out continuously, that is what causes the water to change form back into a substance. This happens when the object that is about to change form moves from the point of the liquid to the melting point of the object.
Types of Heat Transfer
After going through an explanation of the definition, types, formulas of heat, to changes in heat, you can conclude that heat can also move when it meets or comes into contact with other objects. The following are some types of heat transfer.
1. Conduction

Heat transfer by conduction occurs when passing through an intermediate substance such as a metal, but is not followed by a permanent movement of particles in the substance. For example, when you heat one end of a metal, the other end of the metal gets hot as well.
This is due to the conduction of heat from a high temperature to a lower temperature. The heated metal tip will cause the metal particles to create vibrations in other particles connected to them.
Therefore, all metal particles vibrate even if only one end of the metal is heated, which then causes heat transfer. Another example is when the motorcycle exhaust is hot when the motor is turned on.
Conduction also occurs when you hold the fireworks on fire, hold the lid of a pot that feels hot during the cooking process, butter that melts when heated, and so on. The formula for heat transfer for conduction is as follows.
Heat Rate = Q/t = kA. T / x
2. Convection
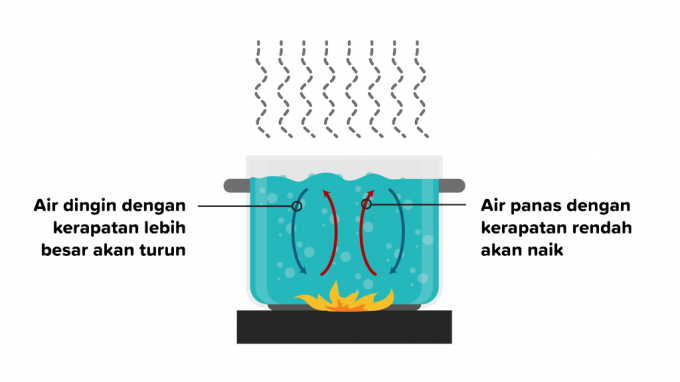
Convection is the transfer of heat in which heat passes through a substance and is followed by the movement of parts of the substance. Heat transfer by convection can occur in liquids or gases, so convection is divided into two as follows.
a. Scientific Convection
Convection is a heat transfer that occurs due to buoyancy without external factors and is influenced by different types of objects. An example case is when heating water, the density of the hot water particles will move away from the fire, then a lower temperature water substance will replace it.
b. Forced Convection
Advertisement
Convection is a heat transfer caused by the influence of external factors, such as pressure, so that the transfer occurs intentionally or forced. That is, heat is forced to move to a place because of the help.
Examples of cases are when a fan that produces cold air to a place that feels hot, the engine cooling system on the car radiator, and so on.
Another application of convection occurs when heating water, where there is an up and down movement of water, green bean seeds that rises and falls when boiled, the process of land and sea breezes, the movement of hot air balloons, chimney smoke factory.
The formula for heat transfer by convection is as follows.
Heat Rate = Q/t = hA. T
3. Radiation

Radiation is the transfer of heat that does not require an intermediate substance or medium. Heat transfer in radiation is not the same as conduction and convection. Displacement in radiation does not always make the two substances touch or meet, because heat can transfer even without an intermediary.
That is, heat will be emitted in all directions by the heat source itself, then flow in all directions that can be achieved. Basically, all objects can emit and absorb heat radiation, but the amount depends on the temperature and color of the substance.
The hotter an object is than the temperature around it, the more heat it radiates to its surroundings. So, if the surface area of a hot object is greater, the heat that will be radiated will be hotter.
An example of radiation cases in everyday life is when you make a bonfire, it will feel warm Due to the fire source being at a certain distance, radiation is felt when the palms become warm when brought near with fire. The heat formula for radiation is as follows.
Heat Rate = Q/t = eσAT4
4. Isolating Heat

Heat has the property of being easily transferred. You can prevent movement from happening, be it by conduction, convection, and radiation. For example by isolating the room; The thermos can keep the water temperature hot or warm, so that heat transfer can be prevented.
Read: Gas Substance
Example of Calorie Problem

The heat formula is a method that you can use to work on the following examples of questions!
1. Example Question 1
An object has a temperature of 5°C and absorbs 1500 J of heat. The temperature of the object changes to 32°C. Calculate the heat capacity of the object!
Solution:
Q = 1500 J
T = 32°C – 5°C = 27°C = 300°K
C = Q / T
C=1500/300
C = 5 J/°K
2. Example Question 2
Calculate the heat required to heat 5 kg of water at 25°C to 105°C if it is known that the specific heat of water is 1000 J/kg°C!
Solution:
m = 5 kg
c = 1000 J/kg°C
T = 105°C – 25°C = 80°C
Q = m. c. T
Q = 5. 1000. 80
Q = 400,000 J
3. Example Question 3
If a water has a mass of 3 kg and is heated from 30°C to 100°C and its specific heat is 1 J/g°C, calculate the amount of heat required for the water!
Solution:
m = 3 kg
c = 1 g/°C = 1000 J/kg°C
T = 100°C – 30°C = 70°C
Q = m. c. T
Q = 3. 1000. 70
Q = 210,000 J
4. Example Question 4
A liquid has a mass of 5 kg. The liquid requires heat of 200,000 J, and is heated from 20°C to 80°C. Calculate the specific heat of the liquid!
Solution:
m = 5 kg
Q = 200,000 J
T = 80°C – 20°C = 60°C
c = Q/m. T
c = 200,000 / 5. 60
c = 200,000 / 300
c = 666.67 J/kg°C
5. Example Question 5
An aluminum has a mass of 5 kg. The initial temperature is 25°C. If the aluminum receives heat as much as 250,000 J and the specific heat is 900 J/kg°C, calculate the final temperature of the aluminum!
Solution:
m = 5 kg
Q = 250,000 J
c = 900 J/kg°C
T1 = 25°C
Q = m. c. T
250.000 = 5. 900. (T2 – 25)
T2 – 25 = 250.000 / 5. 900
T2 – 25 = 250.000 / 4.500
T2 – 25 = 55,56
T2 = 55.56 + 25 = 80.56°C
6. Example Question 6
There are 300 grams of water at a temperature of 25 ° C, will be heated with energy of 1,500 calories. If the specific heat of water is 1 cal/g°C, calculate the temperature of the water after it is heated!
Solution:
m = 300 grams
T1 = 25°C
c = 1 cal/g°C
Q = 1,500 cal
Q = m. c. T
1.500 = 300. 1. (T2 – 25)
T2 – 25 = 1500 / 300. 1
T2 – 25 = 5
T2 = 5 + 25 = 30°C
7. Example Question 7
There are 300 grams of water to be heated from 40°C to 65°C. If the specific heat of water is 1 cal/g°C or 4,200 J/kg°K, calculate:
- A lot of heat is received in calories
- How much heat is received in Joules
Solution:
m = 300 g = 0.3 kg
T = 65 – 40 = 25°C
c = 1 cal/g°C = 4,200 J/kg°K
- Calories in calories
Q = m. c. T
Q = 300. 1. 25
Q = 7,500 calories
- Heat in Joules
Q = m. c. T
Q = 0.3. 4.200. 25
Q = 31,500 calories = 132,300 Joule
Based on the explanation above, the heat formula does not only revolve around releasing and requiring heat, but there is a change in heat, heat transfer, even the specific heat of each substance that vary. Happy practicing and I hope this article is useful!
X CLOSE
Advertisements
ADVERTISEMENT
X CLOSE
