Quantum Number Material: Types, Example Problems (Summary)
Loading...
When explained simply, the quantum number is a number that describes the position of electrons and energy levels or the distance from the atomic nucleus at once, the shape of the orbitals, the orientation of the orbitals, and the spin of electrons contained in the Mechanical Atomic Model Quantum.
Each atom has an orbital, and atomic orbitals have different energy levels. The energy level of the same orbital will have a different energy level if the atoms are different, so the 1s orbital for Hydrogen differs in energy level from the 1s orbital for Helium.
List of contents
Definition of Quantum Numbers

In the wave function, this number has a special meaning to describe the state of the quantum situation. These numbers can describe the state of the electrons in the atom. In 1926, Erwin Schrodinger proposed the Theory of Quantum Mechanics.
This theory explains the structure of the atom. This quantum mechanical atomic model is expressed in a mathematical equation, namely the wave equation. Solving the equation for the hydrogen atom yields a wave function or atomic orbital.
This atomic orbital will describe the situation of the quantum number of electrons in the atom. The square of the wave function means that there is a high probability of getting an electron in a given volume of space around the atomic nucleus.
As with the Heisenberg Uncertainty Principle, the position of electrons in atoms cannot be determined. All that can be known is the position where the electron is most likely to be found.
Types of Quantum Numbers
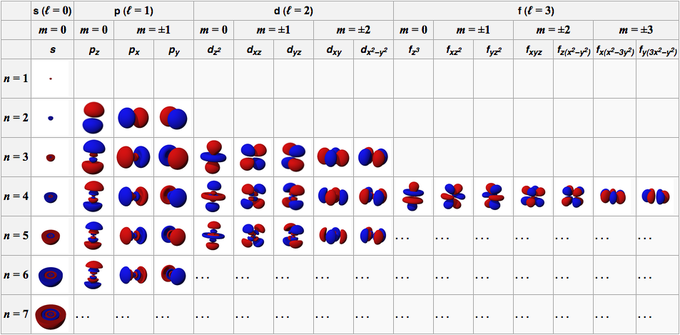
The orbital of an atom is formed from the wave functions that make up the orbital. Under general conditions, the orbital characteristics of an atom are described by four numbers, namely n, l, m, and s.
1. Principal Quantum Number (n)
This number consists of positive integers starting from 1 (one), making the value of n is 1, 2, 3, 4, 5, and so on. This number refers to the shell occupied by an orbital in an atom.
2. Azimuth Quantum Number (l)
This number consists of positive integers starting from 0 (zero), making the value of l 0, 1, 2, 3, 4, 5, and so on. These numbers have their own sign; 0 is the s orbital, 1 is the p orbital, 2 is the d orbital, and 3 is the f orbital.
3. Magnetic Quantum Number (m)
This number consists of integers starting from 0 (zero) to +/- 1, so the number value for each orbital is not the same. For the s orbital, then m is 0 because it is in the s orbital, then l is 0. However, for d orbitals where l is 2, then m is -2, -1, 0, 1, and 2.
4. Spin Quantum Number(s)
If the previous number indicates the orientation of the orbitals, this number describes the spin of the electron which has values of 1/2 and -1/2.
Read: Quantum Mechanics
Orbitals and Quantum Numbers
Each atomic orbital has a unique set of three quantum numbers. These numbers are the principal quantum number (n), azimuth or angular momentum (l), and magnetic (m). These three numbers illustrate several things.
These include describing the energy level of the orbitals, size, shape, and orientation of the possible radial distribution of atomic orbitals. Then, there is the spin number (s), a number that tells you the spin of an electron in an orbital.
Atomic Orbital Shape
There are four forms of atomic orbitals based on their Azimuth number values. The four orbital forms are as follows.
1. s orbitals
The s orbital is an orbital with l = 0. It is spherical in shape, with the atomic nucleus in the center. Since the sphere has only one orientation, all S orbitals have only one value of m, i.e. m = 0. The 1s orbital has the highest electron density or density in the atomic nucleus.

The atomic density then decreases slowly as it moves away from the atomic nucleus. The 2s orbital has two regions of high electron density. From the two regions, there is a spherical node, where the probability of encountering electrons in that region decreases to zero.
The pattern of increasing s orbital vertices will continue with the 4s, 5s, and so on.
2. p. orbitals
The p orbital is an orbital with l = 1. The shape is like a twisted balloon, equipped with two lobes. The lobes are on opposite sides of the atomic nucleus. The atomic nucleus is in the plane of the p orbital node, between the two lobes with high electron density.

The p orbital has three types of spatial orientation, namely Px, Py, and Pz, so there are three possible values of m, namely -1, 0, or +1. The three orbitals are located perpendicular to each other on the x, y, and z axes in Cartesian coordinates. The shape, size and energy are the same.
3. d orbital
The d orbital is an orbital with l = 2. This orbital has five different orientations, giving it five possible values of m, namely -2, -1, 0, +1, or +2. The four d orbitals of which are dxy, dxz, dyx, and d2-y2.
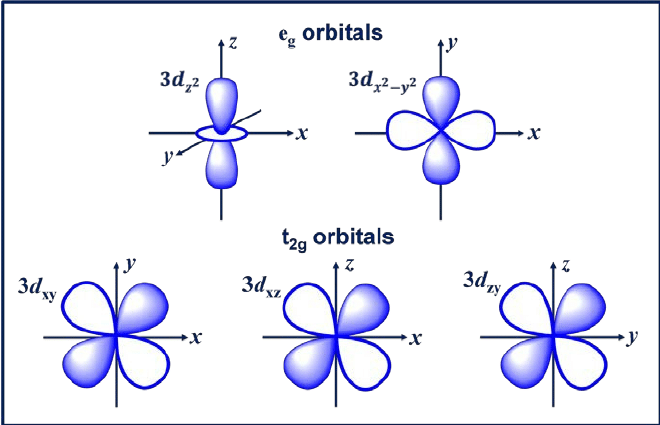
These orbitals have four lobes that are shaped like cloverleaf. The next d orbital is dzw, which has two main lobes on the z-axis and one doughnut-shaped section in the middle.
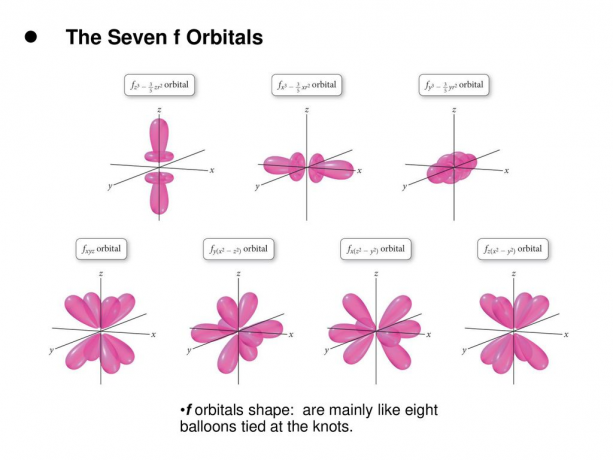
4. f. orbitals
The f orbital is an orbital with l = 3. This orbital has seven types of orientation, just as there are seven possible values of m (2l + 1 = 7). All f orbitals have complex shapes with varying numbers of lobes.
Read: Electron Configuration
Electron Configuration
After you understand how the relationship between the existence of electrons in atoms and orbitals in the Atomic Theory of Quantum Mechanics, Next you will learn about electron configuration, how to arrange electrons in orbitals in atomic shells multi-electron.
1. Aufbau's Rule
Advertisement
In this rule, it is stated that electrons must be filled from lower to higher energy levels. The energy levels of the orbitals can be seen in the arrangement of atoms in the Periodic Table of the Chemical Elements.
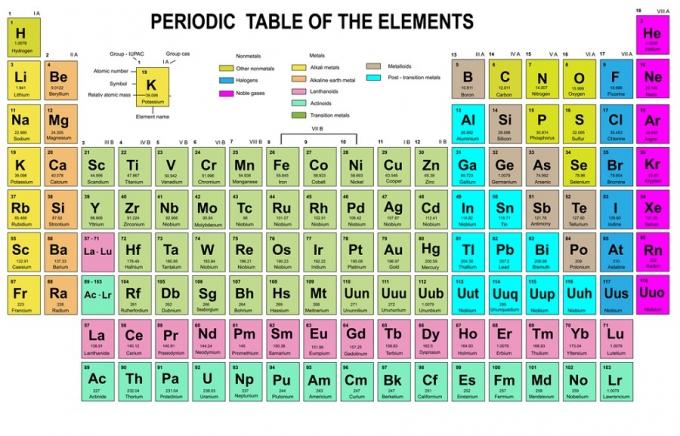
In the table, the bottom left is written in red where there is an s block, blue is for block d, yellow is for block p, and green is for block f. While each row is a skin.
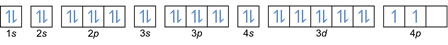
Based on that arrangement, you can see that the order of energy levels is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, and so on.
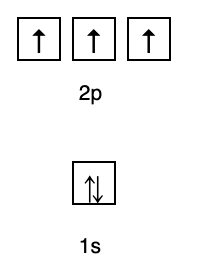
2. Hund Rules
This rule states that if there are orbitals at the same energy level, then the electrons must be filled in parallel until all orbitals in the same energy level are filled with electrons, as shown in Fig following.
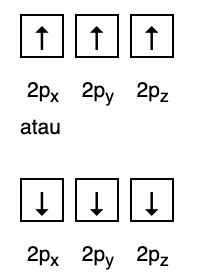
Meanwhile, the following figure does not conform to the filling under Hund's Rule.
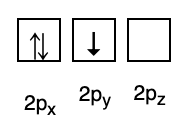
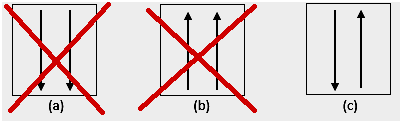
3. Pauli's ban
The Pauli prohibition states that electrons cannot have the same quantum number when filled in an orbital, so there is a spin number (s) with a value of +1/2. The lowest energy is the one with the highest number of paired electrons and parallel spins.
4. Anomaly

According to experiments, there are anomalies in the electron configuration according to the above-mentioned rules. The d subshell is usually half full or full. For example, for 24Cr, its electron configuration: [Ar] 4s1 3d5, is more stable than [Ar] 4s2 3d4.
In addition, the electron configuration for 29Cu: [Ar] 4s1 3d10, more stable than [Ar] 4s2 3d9. Meanwhile, the electron configuration for monatomic ions such as K+, Na+, Ca2+, Br–, S2-, and so on can be determined by the neutral atom first.
For positively charged cations or ions, monatomic Ax+ with a charge of x+, then as many as x electrons are removed from the outer electron shell of the neutral atom A. As for the anion, monatomic By- with a y- charge, as many as y electrons are captured in the lowest energy orbital that is not yet full.
How to Determine Quantum Numbers
Before determining the number, you must first make the electron configuration of the element that you want to find the quantum value for. For example is 16S. Its electron configuration is 1s2 2s2 2p6 3s2 3p4. After that, take the last electron configuration, which is 3p4.
- Based on this configuration, the main number value is 3, because the number 3 represents the size of the orbital or shell.
- p is a subshell of the electron, so it can be obtained under the value of l = 1.
- Because it is in the p subshell, the quantum number will be between -1, 0, or +1. In determining it, first draw the orbital box. You can use the arrows when filling the box.
- Fill in each box with an arrow pointing up, then fill it with an arrow pointing down. The number 4 is the number of arrows that must be filled, to then be obtained like this.
↑↓ ↑ ↑
-1 0 +1
- The fourth (last) arrow is in the -1 box, where the mechanical value is m = -1.
- The up arrow is worth +1/2, while the down arrow is -1/2. The last arrow is the one pointing down, so the value of s = -1/2.
Well, that's how to determine the quantum number. Here are some examples of questions related to this material for you to study.
Read: Physical Change
Examples of Quantum Numbers

Check out the following questions!
1. Example Question 1
Determine the electron configurations and electron diagrams of the following elements and monatomic ions!
- 8O2-
- 20Mg2+
- 26Fe3+
- 27Co
- 32Ge
Solution:
- Electron configuration 8O2:1s2 2s2 2p4 or [He] 2s2 2p4
Electron configuration 8O2–: 1s2 2s2 2p6 or [He] 2s2 2p6 or [Ne] (plus 2 electrons: 2s2 2p4+2)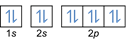
- Electron configuration 20Mg: 1s2 2s2 2p6 3s2 3p6 4s2 or [Ar] 4s2
Electron configuration 20Mg2+: 1s2 2s2 2p6 3s2 3p6 or [Ar] (subtract 2 electrons from outer shell: 4s2-2)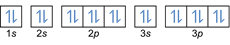
- Electron configuration 26Fe: 1s2 2s2 2p6 3s2 3p6 4s2 3d6 or [Ar] 4s2 3d6
Electron configuration 26Fe3+: 1s2 2s2 2p6 3s2 3p6 3d5 or [Ar]3d5 (subtract 3 electrons from outer shell: 4s2-2 3d6-1)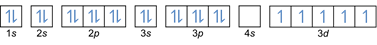
- Electron configuration 27Co: 1s2 2s2 2p6 3s2 3p6 4s2 3d7 or [Ar] 4s2 3d7
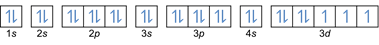
- Electron configuration 32Ge: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2 or [Ar] 4s2 3d10 4p2
2. Example Question 2
The last electron in Ga has a quantum number...
- n = 4; l = 0
- n = 4; l = 1
- n = 3; l = 2
- n = 4; l = 2
- n = 3; l = 1
Solution:
If you look at the Chemical Periodic Table of Elements, the element Ga is in Period IV, giving it an n = 4. Gallium is in Group 13, so the valence electrons are in the p subshell, meaning l = 1.
3. Example Question 3
Below, the quantum number that cannot be occupied by the last electron of Cl atom, is...
- n = 3; l = 1; m = -1; s = -1/2
- n = 3; l = 1; m = 0; s =
- n = 3; l = 2; m = -1; s =
- n = 3; l = 2; m = 1; s = -1/2
- n = 3; l = 1; m = 1; s = 1/2
Solution:
Since the element Cl is in Period 3, then n = 3. Cl is also a Group 17, so its valence electrons are in the p subshell, so l = 1. The value of m can be -1, 0, or +1 because the energies of the three quantum numbers are the same and the order of filling doesn't matter.
For s can also be worth -1/2 or 1/2, so we can not determine m and s with certainty.
4. Example Question 4
An element X3+ has the same electron configuration as the element Ar. So, the ion that has the same configuration as the ion is...
- K+
- Mg2+
- Na+
- Cl+
- F–
Solution:
The element Ar is in Period 3, so the ion having the same electron configuration as Ar or [Ne] 3s2 3p6 will be in Period 4 for cations and in Period 3 for anions. The most appropriate ion is K+.
5. Example Question 5
Determine the quantum number of the element 32Ge!
Solution:
Electron configuration 32Ge: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2 or [Ar] 4s2 3d10 4p2.
The last configuration is 4p2. The energy level is 4, and is in the p subshell, so n = 4 and l = 1. 4p. orbital diagram drawing2 to know the other numbers as follows.
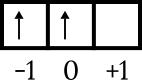
The last electron is in the box orbital 0, the arrow is pointing up, so m = 0 and s = +1/2.
6. Example Question 6
All of the following are allowed quantum numbers, except...
- n = 2; l = 1; m = -1
- n = 3; l = 2; m = 1
- n = 3; l = 3; m = -1
- n = 3; l = 0; m = 0
- n = 3; l = 2; m = -1
Solution:
If the value of the main number (n) is 3, then the maximum azimuth number (l) is n-1 = 3-1 = 2. Therefore, options C with n = 3, l = 3, and m = -1 are not allowed.
To learn quantum numbers, you also need to master some of the atomic numbers of elements that are often used or appear in problems. In addition, you should be familiar with the arrangement of energy levels when constructing electron configurations.
X CLOSE
Advertisements
ADVERTISEMENT
X CLOSE
