Summary of Colloidal Matter: Properties, Types, Examples
Loading...
Hi guys! Did you know that one example of a colloid is butter, a food that is often consumed with bread. But it turns out, there are still many you know another example of colloids that we often find everyday.
What are those? Let's read carefully this article to find out the answer!
List of contents
Definition of Colloid

Colloids are a type of heterogeneous mixture that is formed due to the dispersion of a substance with other substances mixed. Therefore, in a colloidal system there is a dispersion medium and a dispersed phase.
The dispersing medium is a substance that causes an even distribution. Meanwhile, the dispersed phase is a substance that experiences uniform distribution in other substances.
For example, in coconut milk, the granules contained in coconut milk are called the dispersed phase, while the water is called the dispersing medium.
Other examples of colloidal systems are paint, cheese, mayonnaise, jelly, blood, and many more. So, our daily life is closely related to the colloid system.3
Read: Molarity Formula
Colloidal Properties
1. Tyndall Effect

The nature of colloids was first discovered by John Tyndall, a physicist from England. The Tyndall effect is the scattering of light by colloidal particles. When a light beam is directed into a solution, the light will be transmitted.
As a result, we cannot see the light. This is due to the homogeneous nature of the solution and the large size of the colloid molecule.
2. Brown's Motion

Brownian motion is the motion of colloidal particles that is straight, but in an irregular or random direction. When viewed through an ultra microscope, we will see colloidal particles that move in zigzags. This zigzag motion is called Brownian motion which only occurs in liquids and gases.
This is because the movement of colloidal particles of liquid or gas will result in collisions between the colloidal particles themselves. The collision occurred from all directions and resulted in an imbalance that caused a zigzag motion.
The smaller the particle size, the faster Brownian motion occurs. On the other hand, the larger the particle size, the slower Brownian motion occurs.
This explains why Brownian motion is not easy to observe in solution nor is it found in heterogeneous mixtures of solids with liquids or suspensions.
3. Adsorption

Adsorption is the process of adsorption of particles, ions, or other compounds on the surface of colloidal particles due to the large surface area of the particles.
4. Colloidal Coagulation
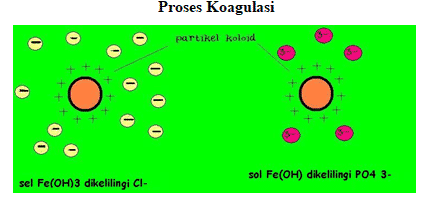
Colloidal coagulation is the clumping of colloidal particles and the formation of a precipitate. As a result, the dispersed substance no longer forms a colloid. These events can easily occur physically, such as cooling and stirring, and chemically, such as the addition of electrolytes.
5. Protective Colloid
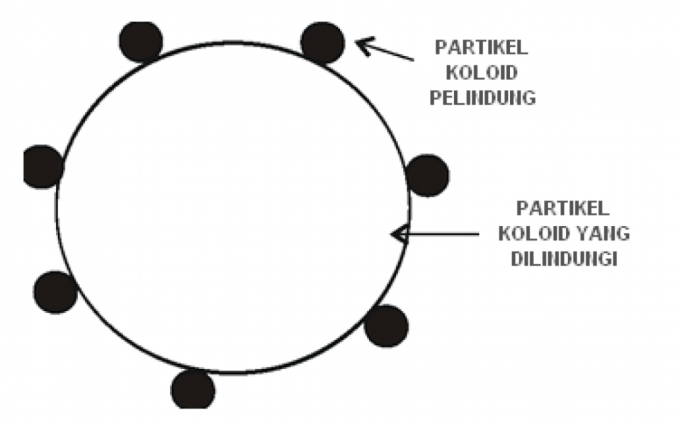
Protective colloids are colloid properties that can protect other colloids from the coagulation process.
6. Dialysis

Dialysis is the process of separating colloids from interfering ions by flowing liquid mixed with colloids through a semipermeable membrane as a filter. This semipermeable membrane can be passed by liquid, but colloid cannot pass.
7. Electrophoresis
Advertisement

The last property of colloids is electrophoresis or the separation of charged colloidal particles using an electric current.
Types of Colloids

Colloids are divided into 8 (eight) types with the following details:
1. Solid sole
The solid sol consists of a solid dispersed phase and a solid dispersing medium. The formation of a solid sol is influenced by pressure and temperature, so that a solid and hard solid is formed. An example of a solid sol is a gemstone (ruby).
2. Sol
The sol consists of a solid dispersed phase and a liquid dispersion medium, so its properties are not easily changed. The difference between the sol and the solid sol lies in the dispersion medium.
3. Solid aerosol
Solid aerosols have a solid dispersed phase in a gaseous dispersion medium. An example of a solid aerosol is vehicle fumes. Therefore, sometimes we feel flickered when exposed to vehicle fumes, because vehicle smoke has a dispersed phase in the form of solids.
4. Aerosol
Aerosol is a type of colloid which has a dispersed phase in the form of a liquid and a dispersion medium in the form of a gas. Aerosols tend not to last long because their constituent substances are easily damaged by air pressure and changes in environmental temperature.
5. Solid emulsion
A solid emulsion consists of a liquid dispersed phase and a solid dispersion medium. For example, gelatin with water as the dispersed phase and the powder as the dispersion medium.
6. Emulsion
Emulsion is a type of colloid in which the dispersed phase and the dispersion medium are liquid. Usually, emulsions are composed of liquids with different polarity compounds. So, the liquids do not mix with each other.
7. Solid foam
Solid foam has a gaseous dispersed phase and a solid dispersion medium. Solid foam can also be called gas dispersed in solid.
8. froth
The foam has a gaseous dispersed phase and a liquid dispersion medium. Foam is also known as gas dispersed in a liquid.
Read: Ionic Bond
Colloid Example Table

| No. | Dispersed Phase | Dispersion Medium | Type | Example |
| 1. | Gas | Liquid | froth | Whipped cream, soap froth |
| 2. | Gas | Congested | Solid foam | Pumice, foam rubber |
| 3. | Liquid | Gas | Aerosol | Fog |
| 4. | Liquid | Liquid | Emulsion | Coconut milk, milk, fish oil |
| 5. | Liquid | Congested | Solid emulsion | Jelly, opal, pearl |
| 6. | Congested | Gas | Aerosol | Dust in the air |
| 7. | Congested | Liquid | Sol | Paint, ink, gold sole |
| 8. | Congested | Congested | Solid sole | Black diamond, colored glass |
Colloidal Making

If earlier we have discussed the meaning of the types of colloids. So, how to make colloids? How to make colloids are as follows:
1. How to condensate
The manufacture of colloids by condensation is by combining solution particles or small particles with larger particles. In short, this method combines existing colloids with smaller particles.
2. Way of dispersion
Dispersion is the process of breaking up large particles (suspension) into small particles (colloids). In this method, there are three stages, namely peptizing, bredig arc, and mechanics.
Read: Quantum Mechanics
Benefits of Colloids

1. Whitening sugar
Sugar that is still dull in color is usually bleached using colloids. The trick is to dissolve sugar in water, then the solution is flowed through a colloidal system of diatomaceous earth.
2. Reducing pollutants in the air
A cottrel precipitator is a device used to absorb smoke and harmful particles from factory exhaust gases. The work of this tool applies the principle of colloidal agglomeration and the nature of the charge, so that the air released will be free from harmful pollutants.
3. Helping the dialysis process for patients with kidney failure
Dialysators or devices used in the dialysis process (dialysis) separate colloidal particles and their solutes.
Examples of Colloidal System Problems

The property of colloids called the ability to scatter light is called...
- Tyndall Effect
- Dialysis
- Brown's Motion
- Adsorption
- Electrophoresis
Answer: A
Mayonnaise is a type of colloid...
- froth
- Aerosol
- Sol
- Emulsion
- Solid foam
Answer: A
Whipped cream is a colloidal system in which the dispersed phase and dispersion medium consist of…
- Liquid and solid
- Solid and gas
- Solid and liquid
- Gas and solid
- Gas and liquid
Answer: E
Well, that's all the discussion and examples of colloids. Finally, it should be noted that colloids are one of the important materials in chemistry subjects. Have a good study!
X CLOSE
Advertisements
ADVERTISEMENT
X CLOSE
