Valence Electrons: Definition, How to Calculate, Example Problems
Loading...
Valence electrons are electrons in the outermost level of an atom that can participate in interactions with other atoms. The valence value can represent an atom so that the interaction value can be seen.
The number of valences in an atom can cause an element to be reactive or inactive. For this reason, it is very important to know the number of valences to be able to know the activity of a related element.
List of contents
Understanding Valence Electrons
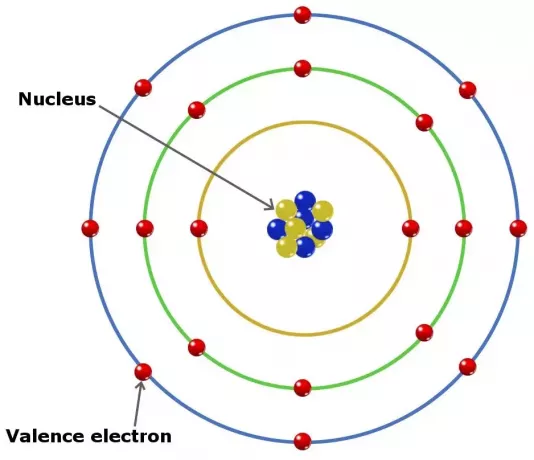
Valence electrons in atoms can play a role in forming a chemical bond in an element. In elements in the main group, valence is the electrons in the outermost shell.
Even so, but not all of these types of electrons are in the outer shell. In some elements belonging to the transition group, the valence is deeper than the outer shell.
Read: Electron Configuration
Valence Electron Table
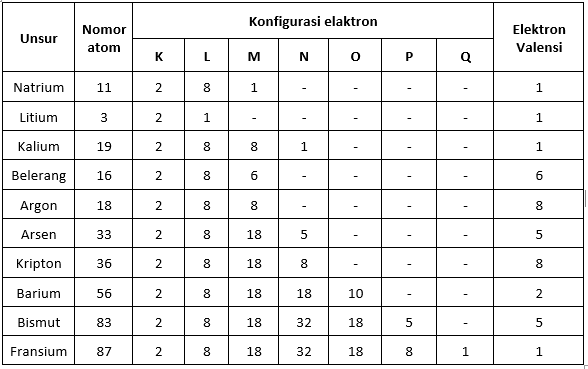
Valence Electrons and the Periodic System of the Elements
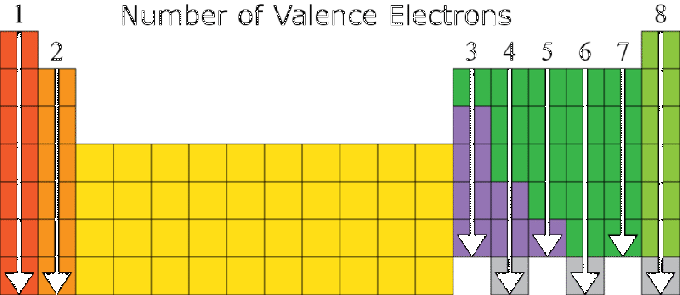
The properties of an element are highly dependent on its electron configuration, especially on its valence number. Elements with the same number of valences generally have similar properties. For this reason, the periodic system of elements is arranged based on increasing atomic number and similarity in the properties of a substance.
There is a relationship between the electron configuration of an element's atom and the position of the element in the periodic system, where:
- The group number is the same as the number of valence values, except for the element He in group VIIIA and the transition group.
- The period number is equal to the number of shells on the electron.
Electrical Conductivity
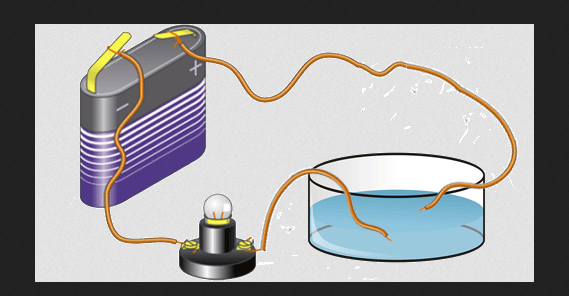
Valence electrons are also responsible for the electrical conductivity of an element. This is what makes the elements can be grouped as non-metals, semiconductors, and metals. Metallic elements generally have high electrical conductivity when in solid state.
In each row of the periodic table, the metal is to the left of the nonmetal. So metals have fewer valences than non-metals.
However, the valence value of metal atoms has a small ionization energy and in the solid state, these electrons are relatively free to leave 1 atom or to combine with other atoms.
Free electrons like this can be moved by the influence of an electric field, their movement can contain an electric current. These electrons are responsible for the electrical conductivity of metals. Examples of good conductors are aluminum, copper, gold, and silver.
Non-metallic elements have low electrical conductivity, so they act as insulators. Elements like this can be seen on the periodic table to the right and have a valence shell that is at least half full.
The ionization energy is said to be large if the electrons cannot leave the atom easily when faced with an electric field. So this kind of element has the ability to conduct very small currents.
Examples of insulating elements include sulfur and diamond. Solid compounds containing metals can also be said to be insulators if the valence of metal atoms is used to form an ionic bond.
Read: Quantum Mechanics
Electron Configuration
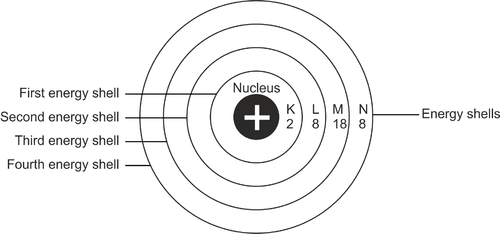
The configuration process can be said to be the arrangement of the distribution of electrons in an atom. Generally, electrons will be in a certain path in an atom. It is determined based on the energy level of an atom. These paths are electron shells.
The first part of the skin layer is denoted by the letter K. The skin layers will continue to L, M, and so on in alphabetical order. The K shell is closest to the atomic nucleus. Each layer of the shell can only accommodate a certain number of electrons.
Advertisement
So if it is full, the electrons will move to the next layer. All layers of skin need to be filled to their maximum capacity.
How to Count Valence Electrons

The position of electrons when they start to fill the atom is starting from the first layer of shell. This layer of skin is closest to the atomic nucleus or is denoted by the letter K. The first layer has the lowest energy level. If the first layer is filled with 2 electrons, it will be continued by filling the next layer called L.
The L shell has a capacity of 8 electrons. If it is full, it will be continued to the next layer of skin gradually. The total capacity of the outermost shell of an atom is 8 electrons.
Elements belonging to the main group, their electron configuration can be determined based on the number of electrons or atomic number according to the following rules.
- The electrons will be fully charged to the maximum limit that can be accommodated by the skin layer.
- If there are still electrons remaining or cannot fill the electron shell layer to the maximum extent, then pay attention to the following provisions.
- If the number of remaining electrons > 32, then the next part of the shell is filled with 32 electrons
- If the remaining electrons < 32, the next shell layer will accommodate 18 electrons;
- If the remaining electrons < 18, the next layer will be filled with 8 electrons.
- If the remaining number is 8, the remaining electrons can fill the next part of the shell.
Read: Physical Change
Examples of Valence Electron Problems

In order to understand the material more deeply, please see some examples and the following discussion.
Example 1
Determine the valence values of the following elements based on their electron configurations.
- 11Na
- 13Al
- 15P
- 18Ar
- 19K
Answer:
- 11Na = 2 8 1
The number of valences of Na = 1
- 13Al = 2 8 3
The number of valences Al = 3
- 15P = 2 8 5
Total valence P = 5
- 18Ar = 2 8 8
The number of valences Ar = 8
- 19K = 2 8 8 1
The number of valences K = 1
Example 2
Create an electron configuration and determine the valence values of the following elements using the noble gas electron configurations.
- 20Ca
- 35br
- 36Cr
- 50Sn
- 86Rn
Answer:
- 20Ca = [Ar] 4s2
The sum of the valence values of Ca = 2
- 35Br = [Ar] 3d10 4s2 4p5
The sum of the valence values of Br = 7
- 36Kr = [Ar] 3d10 4s2 4p6
Sum of valence values Kr = 8
- 50Sn = [Kr] 4d10 5s2 5p2
The sum of the valence values of Sn = 4
- 86Rn = [Xe] 4f14 5d10 6s2 6p6
Sum of valence values Rn = 8
Example 3
Please write the electrons of the elements below and also determine their valence values.
- 10 Ne
- 33 US
- 47 Ag
- 52 Te
- 54 Xe
Answer:
- 10 Ne: 1s2 2s2 2p6
The sum of the valence values of Ne = 8
- 33 Ace: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3
The sum of the valence values of As = 5
- 47 Ag: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d10
The sum of the valence values of Ag = 1
- 52 Te: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p4
The sum of the valence values of Te = 6
- 54 Xe: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6
The sum of the valence values of Xe = 8
Valence electrons can be determined based on the specified path or orbit using the electron configuration. Even though it looks quite complicated, mastering this material is something that needs to be done in order to be able to work on the questions easily.
X CLOSE
Advertisements
ADVERTISEMENT
X CLOSE
