Oxidation Numbers: Definition, Rules of Determination & Examples of Problems
Oxidation Numbers: Definition, Rules of Determination & Examples of Problems – What is the oxidation number and an example? On this occasion About Knowledge.co.id will discuss it and of course about other things that also surround it.
Let's take a look at the discussion in the article below to better understand it
Table of contents
-
Oxidation Numbers: Definition, Rules of Determination & Examples of Problems
- Rules for Determining Oxidation
- Examples of Oxidation Numbers
- Share this:
- Related posts:
Oxidation Numbers: Definition, Rules of Determination & Examples of Problems
Oxidation number or oxidation state is the number of negative and positive charges in an atom, which indirectly indicates the number of electrons that have been accepted or given up to other atoms. Some atoms have only one oxidation state, but there are some atoms that have more than one oxidation state.
If you find the value of an atomic number in a molecule or compound, then you must first know the oxidation state of the atoms of other elements that have general (standard) properties.
Rules for Determining Oxidation
To determine the oxidation number (Biloks) in an ion or other compounds must follow the rules below:
-
The oxidation number of free elements in the form of atoms or molecules of elements is 0 (zero).
The free elements in the form of atoms are:
- The oxidation state of C at C is = 0
- The oxidation state of Ca in Ca is = 0
- The oxidation state of Cu on Cu is = 0
- The oxidation state of Na in Na is = 0
- The oxidation state of Fe in Fe is = 0
- The oxidation number of Al in Al is = 0
- The oxidation state of Ne in Ne is equal to 0
The free elements in the form of molecules are:
- The H oxidation state of H2 is = 0
- The oxidation state of O in O2 is = 0
- The oxidation state of Cl in Cl2 is = 0
- The oxidation state of P in P4 is = 0
- The S oxidation state in S8 is = 0
-
The oxidation state of a metal in a compound is always positive.
In the group 1 metal elements (old system Gol. IA) (Li, Na, K, Rb, Cs, Fr), Its oxidation state is +1.
- The oxidation state of K in KCl, KNO3, or K2SO4 is = +1
In group 2 metal elements (old system group. IIA) (Be, Mg, Ca, Sr, Ba, Ra), Its oxidation state is +2.
- The oxidation state of Mg in MgO, MgCl2, or MgSO4 is = +2
The oxidation numbers (Biloks) of other metal elements are:
- Ag is worth = +1
- Cu is worth = +1 and +2
- Hg is worth = +1 and +2
- Au is worth = +1 and +3
- Fe is worth = +2 and +3
-
The oxidation numbers (Bilox) of monoatomic (for 1 atom) and polyatomic (more than 1 atom) ions are the same in the charge of the ions.
-
The oxidation states of monatomic ions are Na+, Ca2+, Al3+, Cl–, and 02- respectively +1,+2, +3, -1 and -2.
- The oxidation states of polyatomic ions are NH4+, SO42-, PO43-, respectively +1, -2, and -3.
-
-
The oxidation number (Bilox) of group VIA elements (O, S, Se, Te, Po) in binary compounds is -2, and group VIIA elements (F, Cl, Br, I, At) in binary compounds is -1.
-
The oxidation state of the element S in Na2S and MgS is = -2.
- The oxidation state of the element Cl in NaCl, KCl, MgCl2, and FeCl3 is = -1.
-
-
The oxidation number of the element H in its compounds is = +1.
Except for the oxidation state of hydrides (hydrogen compounds with metals) the value = -1.
Because in hydrides, hydrogen exists in the form of the hydride ion, H–. The oxidation state of an ion such as a hydride is the same as the charge on an ion, which is = -1.
- The oxidation state of the element H in H2O, HCl, H2S, and NH3 is = +1.
- The oxidation state of the element H in NaH, CaH2, and AlH3 is = -1.
-
The oxidation number of the element O in its compound is -2, except,
-
The oxidation number of a binary compound in F is = +2.
- The oxidation state of peroxide compounds, such as H2O2, Na2O2 and BaO2, is = -1.
- The oxidation state of superoxide compounds, such as KO2 and NaO2, is = -½ .
- The oxidation number (Biloks) of element O in H2O, KOH, H2SO4 and Na3PO4 is -2
-
-
The sum of the oxidation numbers of the elements in a compound is 0 (zero).
The sum of an oxidation number of elements forming ions or polyatomic compounds is equal to the charge on the polyatomic ion itself.
-
The oxidation number of oxygen (O) in the peroxide compound = -1. Oxidation number of O in non-peroxide compounds = -2.
Example:
O oxidation state in BaO2 = -1.
The Ba atom is a group IIA metal element, so the oxidation state of Ba = +2. The sum of the oxidation states of Ba and O must be 0 (the oxidation rule point 6). Therefore, the oxidation state of O must be -2. Because atom O has index 2, so oxidation state O: index O = -2: 2 = -1. It is proved that the oxidation state of O in BaO2 is -1.
Examples of Oxidation Numbers
Example Question 1
Determine the oxidation number of the elements in bold in the following compounds:
- N2O5
- MnO4–
- Al2(SO4)3
Answer:
The oxidation state will be determined, for example x:
- 1. Charge N2O5 i.e. (2 x oxidation state of N) + (5 x oxidation state of O)
0 = (2x (x)) + (5 x (-2))
0 = 2x – 10
x = +5
So, the oxidation number of the N atom in the compound N2O5 is +5
- 2.Charge of MnO4– is (1 x oxidation number of Mn) + (4 x oxidation state of O) as follows!
-1 is (1 x (x)) + (4 x (-2))
-1 is x – 8
x is +7
So, the oxidation number of the Mn atom in the compound MnO. is4– that is +7
- 3. Al load2(SO4)3 = (2 x oxidation state of Al) + (3 x oxidation state of S) + (12 x oxidation state of O), is:
0 = (2 x (+3)) + (3 x (x)) + (12 x (-2))
0 = 6 + 3x -24
x = +6
So, the oxidation number of the S atom in the compound Al. is2(SO4)3 ie +6.
Example Question 2
Immediately for the Example Problem in Oxidation Numbers, that in ' What is the oxidation number of the C atom in the oxidation number in CH4 with the Oxidation Number Rule H = +1 ?
Answer:
Oxx of H x Number of Atoms H = + '1 x 4 = +4
Oxx C x Number of Atoms C = a x 1 = a
If viewed from the compound is a neutral compound, then the number of oxidation states of the elements in it must be zero, meaning:
a + (+4) = O
a = -4
So the oxidation number of C in the compound CH4 is -4.
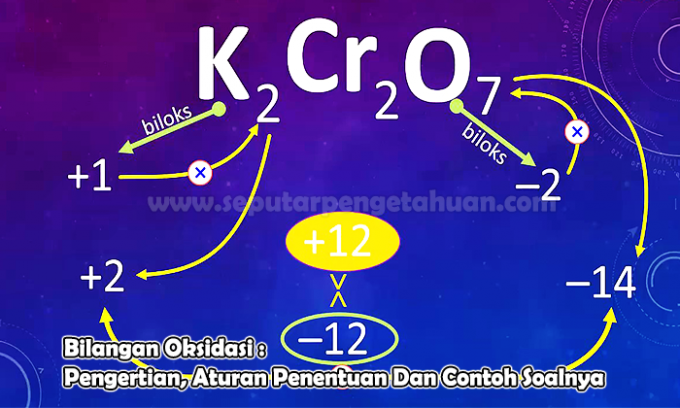
Example Question 3
Determine the oxidation number of the element N in the following compounds:
N2O5
Answer:
The oxidation state will be marked with X
Charge N2O5 i.e. (2 x oxidation state of N) + (5 x oxidation state of O)
0 = (2 x (x)) + (5 x (-2))
0 = 2x – 10
x = +5
So, the oxidation number of the N atom in the compound N. is2O5 that is +5.
That's the review from About Knowledge.co.id about Oxidation Numbers: Definition, Rules of Determination & Examples of Problems, Hopefully it can add to your insight and knowledge. Thank you for visiting and don't forget to read other articles.
Also Read:BMKG: Definition, History, Position and Activities
