Understanding Electrolysis Cells, Types, Reactions, Parts and Examples
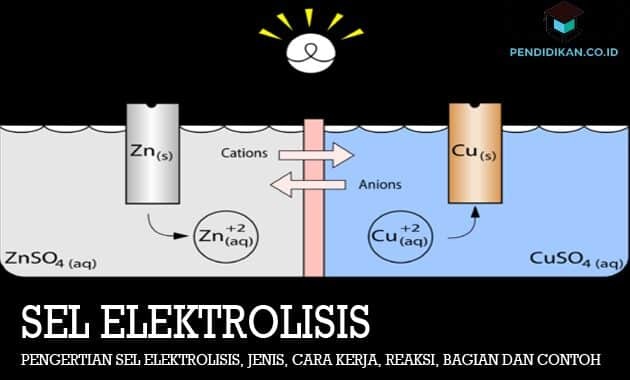
What is Electrolysis Cell
This electrolytic cell is an electrochemical cell in which the electrical energy used to carry out the redox reaction is not spontaneous. The electrolysis reaction can be defined as a decomposition reaction of substances using an electric current. The working principle of an electrolysis cell is to connect the negative pole of the current source in the direction of the cathode and also the positive pole to the anode so that there is an overpotential that causes a reduction and oxidation reaction that is not spontaneous or can take place. The electrons will flow from the cathode to the anode. The positive ions will tend to be attracted to the cathode and are also reduced, while the negative ions will tend to be attracted to the anode and oxidized.
Electrolysis Cell Arrangement
In general, this electrolytic cell is composed of:
- A power source that supplies direct current (dc), for example a battery.
- The anode is the electrode where the oxidation reaction occurs.
- The cathode is the electrode where the reduction reaction occurs.
- Electrolytes are substances that can conduct electricity.
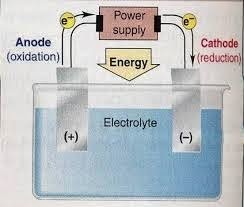
In the picture above, you can see the circuit of a molten NaCl electrolysis cell. The electrolytic cell does not require a salt bridge, as is the case with the Voltaic cell. The electrode used may or may not be an inert electrode such as platinum or graphite which is neither oxidized nor reduced in the cell.
This electrolysis process begins with an electric current flowing in the same direction from a voltage source. Electrons from the negative pole will flow towards the cathode. As a result, the positive Na+ ions in the molten NaCl will be attracted to the cathode and then absorb electrons to be reduced to neutral Na. Meanwhile, the negative Cl− ions in the melt will be attracted to the anode at the positive pole. The Cl− ions will be oxidized to become neutral Cl2 gas by releasing electrons. The electrons will then be flowed to the anode and forwarded to the positive pole of the electric voltage source. So, the redox reaction that occurs in the molten NaCl electrolysis cell can be written as follows.
- Cathode (reduction) = Na+(l) + e− → Na (l)
- Anode (oxidation) = 2Cl−(l) → Cl2(g) + 2e−
- Cell reaction (redox) = 2Na+(l) + 2Cl−(l) → 2Na (l) + Cl2(g)
Electrolysis Cell Part
In the electrolysis cell there are several parts of a circuit that must of course be met for the occurrence of this electrochemical event or occurrence. The main parts of the electrolysis cell include the following;
Electrode

This electrode is a conductor that can or can conduct electric current which in the electrolysis cell, the electrode is divided into two types including anode and cathode.
In the electrolysis process, each anode has its own function, which is like an anode that has functions as a place for oxidation reactions as well as a cathode which has a function as a place for reactions to occur reduction. Both of these reactions complement each other and always occur simultaneously.
The selection of these electrodes can be seen from the value of the reduction potential that is adjusted to the substance to be reacted. Basically, the selected electrode has an inert nature or does not react to the solution or also the reagents used as well as these electrodes have active properties that can or can react with solution.
Examples of inert electrodes that are often used include platinum and carbon, while active electrodes that are often used include copper and nickel.
Electrolyte
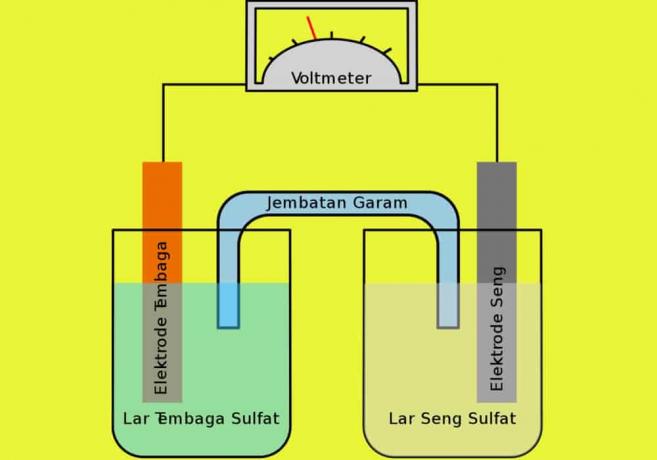
This electrolyte is a solution that is able to conduct electricity or also has a high electrical conductivity. Basically, the electrolyte solution has dissolved ions in a fairly high concentration so from this the movement of ions in the solution has a role in the properties of its conductivity.
The ions in the electrolyte solution will react and experience a good reaction reduction or also oxidation that occurs in the electrode, namely in the presence of an electric current that missed. Examples of electrolyte solutions are CuSO4 and NaCl.
Electric Current Source
This electric current source is also very important for the occurrence of reduction and oxidation reactions. Electric current can be in the form of direct electricity (DC) that will flow through the two electrodes with opposite charges into the electrolyte solution.
The existence of this electric current will certainly cause the movement of electrons from the anode to the cathode which causes the reaction at the anode to occur in an oxidation state and at the cathode reduction occurs. Without an electric current, this reduction and oxidation reaction will not take place because there is no electron transfer in the system.
Electrolysis Cell Type
These electrolytic cells can be classified by the type of electrolyte solution or by the electrodes used. below are the types of electrolytic cells;
Melt/Fmelt Electrolysis Cell
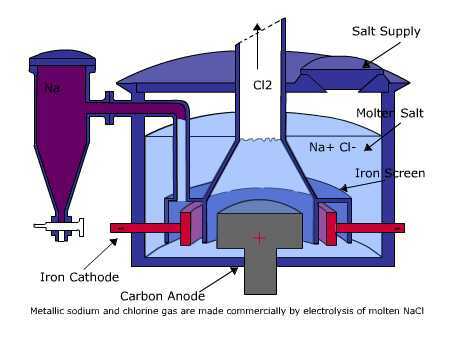
This electrolysis cell is an electrolysis system with the electrolyte in the form of melting or fusion of a substance in the absence of a water solvent.
In this type of electrolyte there are only cations and anions without an H2O molecule. In this type of electrolysis too, the cation will be reduced in the cathode while for the anion it will be oxidized in the anode directly. An example of this type of electrolysis is to use molten NaCl.
Solution Electrolysis Cell
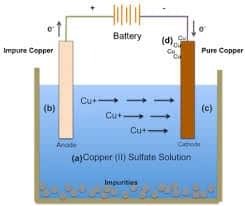
This type is the opposite of the previous type where the electrolyte used is in the form of a solution with water as a solvent. This means that in the electrolyte there is an anion, a cation, and also an H2O. molecule so because of the presence of water it will also be taken into account that there will be competition during the reaction take place.
Electrolysis of this solution is also divided into two types, namely solution electrolysis with inert electrodes and active electrodes. An example of this electrolysis cell is to use an electrolyte solution of CuSO4.
How Electrolysis Cells Work
In an electrolysis cell that has been or has been assembled with 3 complete main components, then the cell can then be run as it should. The working principle of this electrolysis cell is when an electric current is flowed inside the cell electrode, at the anode it will be given a positive charge and while for the cathode it will be charged negative.
Then there will be a movement of electrons as a result of the electric current, the electrons move from the anode to the cathode. As a result, there will be a shortage of electrons in the anode so that the anode will then attract electrons from the solution Electrolytes that contain anions that have a negative charge and will also undergo the oxidation reaction by losing electron.
At this cathode has a lot of negative charge so that it requires a positive charge to be able to neutralize it, by Therefore this cathode tends to attract cations in the electrolyte so that the cations will then be reduced by accepting electron.
Electrolysis Reaction
In general, the electrolysis of molten ionic compounds involves simpler redox reactions. This is because in the absence of water, the cations will be reduced at the cathode and the anions will be oxidized at the anode. For example, in the electrolysis of molten MgBr2, the Mg2+ ions will be reduced at the cathode to form Mg metal and also Br− ions will be oxidized at the anode to form Br2 gas.
However, if the electrolysis reaction takes place in a solution system, there are several competing redox reactions so that from this the reactions tend to be rather complex. Some of the factors that determine the electrolysis reaction of this electrolyte solution include the following.
1. Sessions in the electrolyte solution
This reduced species is the species with a more positive reduction potential
this oxidized species is the species with a more negative reduction potential (more positive oxidation potential)
2. The nature of the electrode material, inert or active
This inert electrode is an electrode that is not involved in the redox reaction of electrolysis. Examples are such as: platinum (Pt), gold (Au), and graphite (C).
This active electrode is an electrode that can or may be involved in an electrolytic redox reaction. Examples are such as: copper (Cu), chromium (Cr), and nickel (Ni).
3. The additional potential (overpotential) given
The overpotential is needed to be able to exceed the interaction on the electrode surface which generally occurs when electrolysis produces gas.
Based on the standard electrode potential data, the electrolysis reaction of the electrolyte solution under standard conditions can be predicted by following the following provisions.
For example, we can observe the difference between the electrolysis of the AgNO3 solution with the graphite electrode as well as the following silver (Ag) electrode.
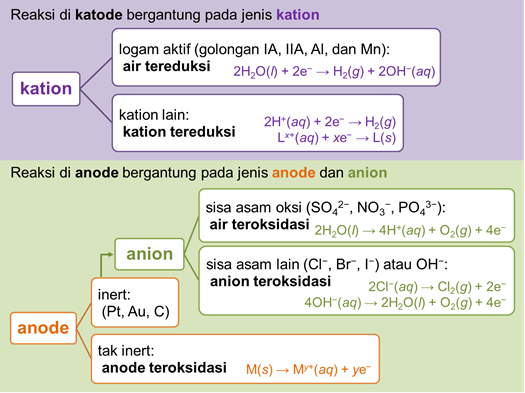
a. Electrolysis of AgNO3 solution with graphite electrode
At the cathode, the species that undergoes this reduction is Ag+. This is because Ag is not an active metal whose reduction potential is more negative than the reduction potential of water.
Cathode: Ag+(aq) + e− → Ag(s)
At the anode, the graphite electrode is an inert electrode so that it is not oxidized. This NO3− species is an oxyacid residue which is difficult to oxidize, as a result, water will be oxidized.
Anode: 2H2O(l) → 4H+(aq) + O2(g) + 4e−
b. Electrolysis of AgNO3 solution with silver electrode
At the cathode, the species that undergoes reduction is Ag+. The reduced species at the cathode does not depend on the electrode used, but only on the type of cation in the electrolyte solution.
Cathode: Ag+(aq) + e− → Ag(s)
At the anode, the Ag electrode is not an inert electrode so it will be oxidized.
Anode: Ag(s) → Ag+(aq) + e−
Examples of Electrolysis Cell Problems and Discussion
Write the electrolysis reaction below.
a. electrolysis of CuSO4 solution with copper electrodes
b. electrolysis of KI solution with graphite electrode
c. electrolysis of molten CaCl2 with platinum electrodes
Answer:
- CuSO4(aq) → Cu2+(aq) + SO42−(aq)
This Cu is not an active metal, so from this the Cu2+ cation will be reduced at the cathode. Therefore, the copper (Cu) electrode is not an inert electrode, so the Cu anode will be oxidized.
Cathode: Cu2+(aq) + 2e− → Cu(s)
Anode: Cu(s) → Cu2+(aq) + 2e−
Cell reaction: Cu(s) anode → Cu(s) cathode
- KI(aq) → K+(aq) + I−(aq)
This K is an active metal, so from that water will be reduced at the cathode. Therefore, this graphite electrode includes an inert electrode and the I− anion does not include the rest of the oxy acid, therefore the I− anion will be oxidized at the anode.
Cathode: 2H2O(l)+2e− → H2(g)+2OH−(aq)
Anode: 2I−(aq) → I2(g)+2e−
Cell reaction: 2H2O(l) +2I−(aq) →H2(g)+2OH−(aq)+I2(g)
- CaCl2(l) → Ca2+(l) + 2Cl−(l)
For the electrolysis of the molten CaCl2 ionic compound with a platinum electrode (this includes inert electrode), the Ca2+ cation will be reduced at the cathode and the Cl− anion will be oxidized at the anode.
Cathode: Ca2+(l) + 2e− → Ca(s)
Anode: 2Cl−(l) → Cl2(g) + 2e−
Cell reaction: Ca2+(l) + 2Cl−(l) → Ca(s) + Cl2(g)
Thus the explanation of the definition of an electrolytic cell, its types, how it works, reactions, parts and examples, hopefully what is described can be useful for you. thank you
See AlsoDefinition of Anabolism
See AlsoUnderstanding Startups
See AlsoUnderstanding, Characteristics and Types of Political Culture in General According to Experts
